References: 1. Mebarki, M., et al., Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther, 2021. 12(1): p. 152. 2. Soder, R.P., et al., A Phase I Study to Evaluate Two Doses of Wharton's Jelly-Derived Mesenchymal Stromal Cells for the Treatment of De Novo High-Risk or Steroid-Refractory Acute Graft Versus Host Disease. Stem Cell Rev Rep, 2020. 16(5): p. 979-991. 3. Ding, D.C., W.C. Shyu, and S.Z. Lin, Mesenchymal stem cells. Cell Transplant, 2011. 20(1): p. 5-14. 4. Racchetti, G. and J. Meldolesi, Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered with Extraordinary Success. Biomedicines, 2021. 9(6). 5. Ayala-Cuellar, A.P., et al., Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol Ther (Seoul), 2019. 27(1): p. 25-33. 6. Ankrum, J.A., J.F. Ong, and J.M. Karp, Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol, 2014. 32(3): p. 252-60. 7. Kalaszczynska, I. and K. Ferdyn, Wharton's jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int, 2015. 2015: p. 430847. 8. Ullah, I., R.B. Subbarao, and G.J. Rho, Human mesenchymal stem cells - current trends and future prospective. Biosci Rep, 2015. 35(2). 9. Stefanska, K., et al., Human Wharton's Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J Clin Med, 2020. 9(4). 10. Li, C., et al., Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep, 2019. 39(5).
Blog
What We Understand About MSCs in Umbilical Cord Tissue and Wharton’s Jelly
Once a hot topic only theorized in academia, stem cells have taken life sciences and biomedical development by storm. This is due to the well-characterized biological processes that allow them to perform important and complex roles, both in vivo and in vitro. In modern research, the most promising source of stem cells are mesenchymal stem cells, which are abundant in umbilical cord tissue and Wharton’s jelly[1, 2].
MSCs are a class of pluripotent stem cells originating from the mesoderm, or middle germ layer[3]. MSCs inhabit the stroma of several tissues, where they exist as a self-replenishing cell pool. They can reproduce and incorporate into the tissues as part of endogenous repair processes. They also release many beneficial factors into the local microenvironment, such as trophic factors and extracellular vesicles[3, 4]. This supports the regenerative actions of cells and tissues[5]. Because of this, MSCs have been investigated for health purposes.
MSCs are extremely useful in many applications because of their unique properties. They lack histocompatibility complexes and are therefore largely ignored by the system, bypassing the issue of tissue transplant rejection[6]. In addition, they can take on a wide range of cell fates, allowing for multiple research and clinical opportunities[3, 5]. Unlike embryonic stem cells, they are not tumorigenic [7]. Moreover, the use of MSCs is not burdened with the same ethical issues since they are not derived from fetal tissue.
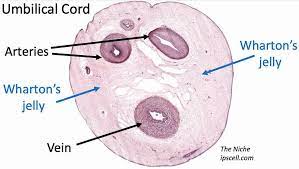
MSCs were first identified in mouse bone marrow and are present in most tissues[8]. However, their relative abundance and accessibility vary by source. They have been gathered from bone marrow and adipose tissue, but recently, Wharton’s jelly and umbilical cord tissue have been used as MSC sources [1, 9]. This is because they culture easily and are particularly easy to harvest. Since both are collected from what would otherwise be medical waste, MSCs from these sources are plentiful[1, 9].
Research into MSC therapy originally focused on cell differentiation and tissue integration, but this was met with mixed results. However, further work discovered that they release a potent milieu of trophic factors into the microenvironment, stimulating regenerative effects[5]. They can also transfer mitochondria, a process that can promote tissue regeneration by reducing oxidative stress and metabolic derangement[10].
Research into the clinical applications of MSCs is booming. As of 2019, over 800 clinical trials were studying their therapeutic role, with many more being implemented since[1, 2, 9, 10]. Umbilical cord-derived MSCs have been studied in clinical trials for potential treatments in immune diseases and other conditions wherein inflammation plays a pivotal role[1]. This includes type I diabetes mellitus, lupus erythematosus, multiple sclerosis, Crohn’s disease, and more, with promising results. Likewise, Wharton’s jelly-derived MSCs have been used in such conditions as HIV, amyotrophic lateral sclerosis, active and refractory lupus, type II diabetes, and leukemia[9].
At Leo Corps, Inc., we are dedicated to providing the highest quality MSCs and other cell products to accelerate preclinical and clinical research and development. In addition, we provide consulting and licensing services and provide accurate hematology analysis with a quick turnover. If your projects rely on quality individualized cell products, contact us today!