1. Ayala-Cuellar, A.P., et al., Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol Ther (Seoul), 2019. 27(1): p. 25-33. 2. Ding, D.C., W.C. Shyu, and S.Z. Lin, Mesenchymal stem cells. Cell Transplant, 2011. 20(1): p. 5-14. 3. Racchetti, G. and J. Meldolesi, Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered with Extraordinary Success. Biomedicines, 2021. 9(6). 4. Mohammadalipour, A., S.P. Dumbali, and P.L. Wenzel, Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front Cell Dev Biol, 2020. 8: p. 603292. 5. Liu, D., et al., Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther, 2021. 6(1): p. 65. 6. Nicolson, G.L., Mitochondrial Dysfunction and Chronic Disease: Treatment With Natural Supplements. Integr Med (Encinitas), 2014. 13(4): p. 35-43. 7. Kumar, A., Editorial (Thematic Selection: Mitochondrial Dysfunction & Neurological Disorders). Curr Neuropharmacol, 2016. 14(6): p. 565-6. 8. Ding, W.X. and X.M. Yin, Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem, 2012. 393(7): p. 547-64. 9. Chen, G., G. Kroemer, and O. Kepp, Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front Cell Dev Biol, 2020. 8: p. 200. 10. Li, C., et al., Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword. Biosci Rep, 2019. 39(5).
Blog
Mitochondrial Transfer and MSCs
Research in mesenchymal stem cells (MSCs) has boomed in recent years. Originally, MSCs were used to target regeneration through differentiation and integration into target tissues[1, 2]. However, this endeavor was met with mixed results[1-3]. Despite some setbacks, this research established that MSCs play important roles in endogenous regenerative processes that could potentially be leveraged for use in advanced cell therapies.
Researchers found that MSCs release many trophic and immunomodulatory factors into the microenvironment. In addition, they release a plethora of extracellular vesicles that play important roles in exocrine signalling. Recently, attention has turned to their role in mitochondrial transfer, a process associated with tissue regeneration[4].
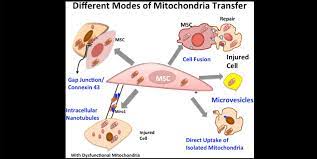
Mitochondrial transfer is the exchange of mitochondria between cells. It was first discovered that cells could transfer mitochondria in 2004, wherein researchers found that the organelles moved between cells through tunneling nanotubules. Later research found that MSCs transferred their comparatively healthy mitochondria to cells with dysfunctional mitochondria[5].
Mitochondrial dysfunction is at the root of many degenerative conditions and disease states. When mitochondrial function breaks down, energy output drops, and the production of damaging reactive oxygen species (ROS) ramps up. While oxidative phosphorylation produces a level of ROS that promotes important processes in healthy conditions, ROS damages cellular and mitochondrial components at high levels[6]. This causes a runaway forward feedback loop that leads to progressive cellular, tissue, and systemic damage. Mitochondrial dysfunction particularly impacts neuronal health, as neurons are particularly sensitive to metabolic collapse and oxidative damage[7].
As a result, cells must have repair mechanisms to preserve mitochondrial integrity. Cells typically perform this via a process called mitophagy[8]. As mitochondria split and fuse, they share their molecular contents to offset damaged components. Daughter mitochondria with damage that is too far gone are flagged for autophagic degradation. However, mitophagy can be too little, too late when mitochondrial damage has progressed too far[8]. Failure of this process is associated with many age-related conditions, such as neurodegenerative diseases, cancer, and cardiovascular disease[9].
When mitophagy is insufficient to rescue mitochondrial dysfunction, some cells can engage in mitochondrial transfer. As neighboring cells release ROS and damage associated molecular patterns (DAMPs), collections of molecules released from damaged and dying cells, MSCs begin to transfer their functional mitochondria to the cells in an attempt to stem the tide of further harm[4, 5]. By transferring mitochondria, MSCs can help stabilize cellular metabolism of their injured neighbors and abate the damage caused by runaway mitochondrial dysfunction. Through this process, it is thought that mitochondrial transfer is one major underlying mechanism of the regenerative power of MSCs, be they endogenous or from external sources.
However, like any biological process, whether mitochondrial transfer is “bad” or “good” depends on the context. Tumor cells have been found to recruit other cells for metastasis, in part, by exchanging defective or mutated mitochondria[10]. As a result, unregulated mitochondrial transfer can cause harms, but this is predominately in the context of cancer. MSC-related mitochondrial transfer is not associated with tumorigenesis.
Mitochondrial transfer is a cutting-edge field of research that has the power to accelerate clinical and preclinical investigations. At Leo Corps, Inc., we provide the highest quality MSCs and cell products to explore mitochondrial transfer and other regenerative processes. If your research could benefit from investigating this exciting line of research, contact us today to find out how we can help!