By Andrew Proulx MD
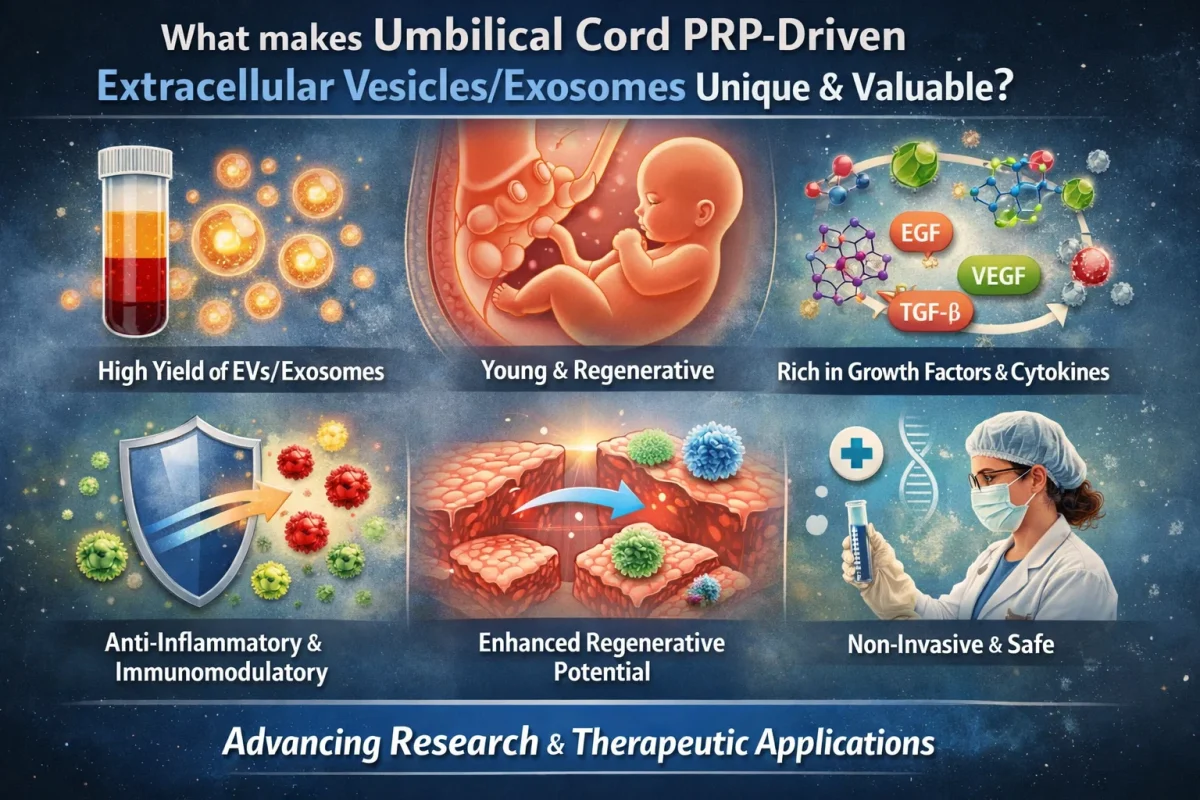
Regenerative medicine is a fast-paced area of research with near-unlimited potential for innovation, discovery, and significant clinically relevant breakthroughs.1 One such area of innovation involves combining the significant regenerative potential of extracellular vesicles (EVs) with that of umbilical cord-derived platelet-rich plasma (PRP).2
Cell-Free Approach
While the anti-inflammatory, immunomodulatory, and secretory properties, as well as the ability to differentiate, make mesenchymal stem cells (MSCs) a potent vector for regenerative medicine, MSCs are not without their limitations.3 These limitations have spurred work on cell-free approaches to tissue regeneration. One such approach is the use of exosomes, particularly umbilical cord PRP-driven exosomes (UC-PRP-Exos).2 This has largely followed from the finding that extracellular vesicles (EVs, especially exosomes) are solely responsible for the therapeutic effect of MSCs.4 This includes modifying the phenotype and function of cells.
Umbilical Cord as a Source for PRP
Umbilical cord blood-derived PRP (UCB-PRP) has advantages over peripheral blood-derived PRP. UCB-PRP appears to have more growth and anti-inflammatory factors compared with autologous PRP from adult peripheral blood, and may have higher potency due to the unique composition of its paracrine biochemicals.5,6 UCB-PRP also has a cost and availability advantage, as cord blood banks are now prevalent worldwide, storing cord blood for hematologic purposes.6 UBC-PRP is generally considered a waste product from therapies that use cord blood and is easily collected during these procedures.6
Low Immunogenicity
UC-PRP-Exos are derived from immunologically privileged neonatal tissue and have low immunogenicity; therefore, they are an excellent vehicle for allogenic applications.7 This eliminates the need for collecting patients’ own blood for each treatment and makes them an excellent medium for researchers, attenuating the cost, supply, immunological, and ethical concerns related to the use of limited blood products.
Tissue Access
UC-PRP-Exos are acellular nano-particles with a lipid bilayer structure, so they can readily cross biological barriers (including the blood–brain, skin mucosal, and placental barriers) and can be modified to improve their mobility efficiency.8 They can cross barriers that cells or platelets cannot, and they protect their cargo until they reach their target tissue.
Multifunctionality
Their near-unlimited tissue access and their diverse paracrine cargo give UC-PRP-Exos a multifunctional role at the target tissue. The same treatment may alleviate inflammation, promote angiogenesis, support wound healing, and initiate cell differentiation.9
Unanswered Questions and Room for Innovation
UC-PRP-Exos represent a rich avenue of inquiry for research and development. Their effectiveness in a wide range of clinical applications has been demonstrated, and they have vast potential for further inquiry.5,8 Exosomes in general can be modified and have already been used as carriers of drugs, nucleic acids, and protein receptors for specific applications.8 Taken together, their allogenic low-immunogenicity, availability, lack of ethical burden, biological penetration, rich paracrine profile, and modifiability make UC-PRP-Exos an as-yet underexplored but potent avenue of inquiry for regenerative medicine in a wide variety of human tissues and conditions.
References:
1. Tatullo M, Zavan B, Piattelli A. Critical overview on regenerative medicine: New insights into the role of stem cells and innovative biomaterials. Int J Mol Sci. 2023;24(9):7936. doi:10.3390/ijms24097936
2. Chang Y-H,i Wu K-C, Ding D-H. Enhancing the therapeutic potential of human umbilical cord mesenchymal stem cells for osteoarthritis: The role of platelet-rich plasma and extracellular vesicles. Int J Mol Sci. 2025;26(8):3785. doi:10.3390/ijms26083785
3. Flamant S, Loinard C, Tamarat, R. MSC beneficial effects and limitations, and MSC-derived extracellular vesicles as a new cell-free therapy for tissue regeneration in irradiated condition. Environ Adv. 2023;13:100408. doi:10.1016/j.envadv.2023.100408.
4. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. https://doi.org/10.1186/s13287-018-0791-7
5. Tang Y, Li Y, Chen H, et al. Application of cord blood-derived platelet-rich plasma in the treatment of diseases. J Int Med Res. 2024;52(7):3000605241263729. doi:10.1177/03000605241263729
6. Subiran C, Kristensen SG, Andersen CY. Umbilical cord blood–derived platelet-rich plasma: A clinically acceptable substitute for fetal bovine serum? Fertil Steril. 2021;115(2):336-337. doi:10.1016/j.fertnstert.2020.10.027
7. Tong TH, Do XH, Nguyen TT, et al. Umbilical cord blood-derived platelet-rich plasma as a coating substrate supporting cell adhesion and biological activities of wound healing. Eur J Med Res. 2025;30(1):145. doi:10.1186/s40001-025-02388-8
8. Jin X, Zhang J, Zhang Y, et al. Different origin-derived exosomes and their clinical advantages in cancer therapy. Front Immunol. 2024;15:1401852. doi:10.3389/fimmu.2024.1401852
9. Zhang Y, Yi D, Hong D, et al. Platelet-rich plasma-derived exosomes enhance mesenchymal stem cell paracrine function and nerve regeneration potential. Biochem Biophys Res Commun. 2024;699:149496. doi:10.1016/j.bbrc.2024.149496.